In this work, we identify a discrete NMT, LcsG, from the biological control fungus Purpureocillium lilacinum PLBJ-1 that catalyzes the incorporation of a unique moiety located at the terminus of an NRP26. Deleting the lcsG gene led to the disappearance of leucinostatins with methylated terminus. Subsequent in vitro enzyme activity assays and structural elucidation of the products demonstrated that LcsG is involved in the iterative methylation of terminal-free amines in leucinostatins. Moreover, structure‒function relationship analysis provided a probable insight into the catalytic mechanism of LcsG. Additionally, we obtained new leucinostatins from the LcsG-catalyzed reaction, that can inhibit the growth of the human pathogen Cryptococcus neoformans and the plant pathogen Phytophthora infestans.
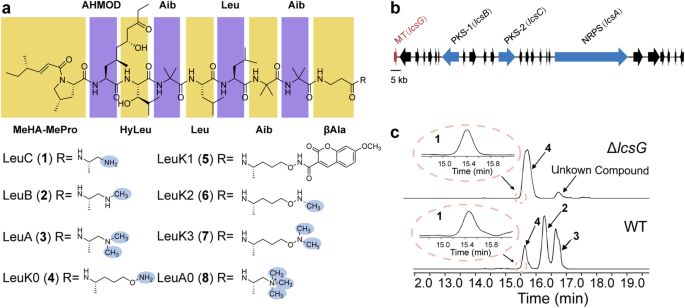
Our previous studies demonstrated that the P. lilacinum PLBJ-1 strain harbors the leucinostatin biosynthesis-related gene cluster (BGC), and the lcsG gene, which was predicted to function as a methyltransferase attracted our interest (Fig. 1b). LcsG is equipped with the Methyltransf_2 domain, which is commonly found in O-methyltransferases (OMTs). The top hit in its pBLAST search shared 31.65% sequence identity with an OMT called VdtC (A0A443HJY8.1). However, despite this prediction, we did not find any O-methylated units in leucinostatins. This inconsistency prompted us to investigate the function of LcsG.
To determine the function of LcsG in leucinostatin biosynthesis, we constructed deletion mutants (ΔlcsG) and overexpression mutants (OElcsG) of P. lilacinum PLBJ-1 (Supplementary Figs. 1-3). Following growth on a productive medium and production extraction, LC‒MS analysis was performed, revealing obvious differences between the deletion mutant and wild-type strains (WT) (Fig. 1c); in contrast, there were no obvious differences between the overexpression mutants (OE1, OE2, which correspond to strains overexpressing the lcsG gene) and the WT (Supplementary Fig. 3). LC‒MS analysis of the WT extracts revealed that the m/z [M + H] values of the four peaks (1-4) were 1190.8133, 1218.8439, 1204.8247, and 1234.8388, respectively (Supplementary Fig. 4). High-resolution electrospray ionization mass spectrometry (HRESI-MS-MS) analysis (Supplementary Table 1 and Supplementary Fig. 5) confirmed that peaks 1-3 were attributed to the LeuC, LeuB, and LeuA, respectively. Based on the HRESI-MS-MS results and the same molecular weight obtained, peak 4 was initially presumed to be leucinostatin K (LeuK), a compound isolated from Paecilomyces lilacinus (synonym Purpureocillium lilacinum). However, the NMR analysis suggested that peak 4 was not the known compound LeuK but rather a compound derived from LeuC whose C-terminus was -NH-CH-CH-OH or -CH-CH-O-NH. As its structure was not fully consistent with that of LeuC, the compound has not been previously reported, we designated it as leucinostatin K0 (4, LeuK0) since the name “K0” was unclaimed (Supplementary Figs. 6-8). A specific N-hydroxysuccinimide (NHS)-ester reaction was employed to verify the free amine in LeuK0, and then the product compound LeuK1 (5) with NHS ester labeling was detected (Supplementary Fig. 9), which confirmed the structure of LueK0 (4) as shown in Fig. 1a. The deletion of lcsG led to the abolishment of LeuB and LeuA, both of which have methylated termini, suggesting that the deletion blocked the formation of the methylated C-terminal amines. Moreover, LeuK0 and an unidentified compound that shared the same C-terminus (Supplementary Table 1) persisted in the deletion mutant (Fig. 1c). Therefore, LcsG was inferred to play an essential role in the biosynthesis of terminal amines of leucinostatins.
Sequence analysis suggested that LcsG is an S-adenosyl–methionine (SAM)-dependent methyltransferase. To clarify its biochemical function, we expressed lcsG in E. coli ArcticExpress (DE3) and purified the recombinant protein LcsG, which was tagged with His at both the N and C termini, using nickel affinity chromatography (Fig. 2a). Obtaining pure LeuB and LeuC in the laboratory is exceptionally difficult. The enzymatic activity was assayed by using LeuK0 (4) and LeuA (3) as the substrates. The S-adenosyl–homocysteine (SAH) and three new product peaks, LeuK2 (6), LeuK3 (7) and LeuA0 (8), were detected only in the presence of the substrate, LcsG, and SAM (Supplementary Fig. 10 and Fig. 2b). In contrast, omitting LcsG or SAM resulted in no product formation. LC‒MS analysis confirmed that the [M + H] values of the 6-8 ions were 1248.8533, 1262.8711, and 1232.8595, respectively (Supplementary Fig. 11). These molecular weights indicated that LeuK2 (6) and LeuA0 (8) are the methylated products of LeuK0 (4) and LeuA (3), respectively, and that LeuK3 (7) is the dimethylated product of LeuK0 (4). These results indicated that LcsG is likely a SAM-dependent methyltransferase.
The kinetic activity of LcsG was analyzed to gain further insight into its methylation activity. We conducted several single-factor enzymatic assays. The ethyl acetate (EtOAc) extracts of the WT PLBJ-1 strain cultures, which inherently contained LeuB (2), LeuA (3), and LeuK0 (4), served as substrates. We also established a control group without the addition of LcsG and employed LC‒MS analysis to quantify each component in the experimental and control groups. The variations in component levels between the experimental and control groups were compared to characterize the changes induced by LcsG catalysis. Positive values indicate that a component’s level in the experimental group is greater than that in the control group, suggesting that a component accumulates during catalysis. Conversely, negative values suggest that the level of the component in the experimental group was lower than that in the control group, indicating that a component is consumed during catalysis. The time dependency of the LcsG-catalyzed reaction showed that LeuA0 (8) and LeuK3 (7) accumulated nearly linearly with time and appeared almost immediately after the reaction started. LeuB (2) and LeuK0 (4) were nearly completely consumed after the beginning of the reaction, while the amount of LeuA (3) and LeuK2 (6) increased early on and decreased (Fig. 2c). These results suggested that LcsG is an iterative methyltransferase, and the reaction sequences could be LeuB-LeuA-LeuA0 and LeuK0-LeuK2-LeuK3. The optimal pH and temperature for producing the final products LeuA0 and LeuK3 were determined (Supplementary Fig. 12), followed by measurements of the initial rates at substrate concentrations ranging from 0-200 μM. The initial rate data were measured by LC‒MS and fitted to the Michaelis‒Menten equation to determine the kinetic parameters (Fig. 2d and Supplementary Fig. 13). The K/K values obtained for LeuA (3) and LeuK0 (4) were 65.39 s M and 32.94 s M, respectively.
Next, we performed HRESI-MS-MS analysis to elucidate the structures of compounds LeuK2 (6), LeuK3 (7) and LeuA0 (8), we turned to HRESI-MS-MS analysis. The m/z values of the fragments of each leucinostatin are presented in Supplementary Table 1. Comparisons of the MS-MS data of LeuA0 (8) with those of LeuA (3) and LeuB (2) revealed that the spectrum of LeuA0 (8) showed remarkably similar fragments to those of LeuA (3) and LeuB (2) (Supplementary Fig. 14). Specifically, they shared one fragment with a m/z value of 1173. This ion was deduced to be the [M + H] ion of fragment C10, indicating the possible methylation site of the C-terminal amine. Therefore, LeuA0 (8) was concluded to be a trimethylammonium compound in which the terminal amine carried a positive charge and three methyl groups. This predicted structure is identical to a previously identified structure, which was obtained by treating LeuA (3) with methyl iodide. This reaction afforded the same product as the enzymatic reaction (Fig. 3a), confirming the structure of LeuA0 (Fig. 3c). Combined with its molecular weight, LeuA0 (8) was assigned the molecular formula CHNO.
Treating LeuK0 (4) with methyl iodide produced LeuK2 (6) and LeuK3 (7) as shown in Fig. 3b. Based on their HRESIMS data, LeuK2 (6) and LeuK3 (7) have molecular formulas of CHNO and CHNO, respectively. MS-MS experiments revealed similar fragments to LeuK0, with differences in Y-type fragments. Notably, the Y-type fragments in LeuK2 (6) are +14 mass units larger than those in LeuK0 (4). For example, m/z values of 190 and 204 correspond to [M + H] ions of Y2 fragments in LeuK0 and LeuK2 (6), respectively. Furthermore, LeuK2 (6) and LeuK3 (7) produced an ion at m/z 1173 (C10), indicating that the terminal amine of LeuK2 was methylated LeuK2 (6) (Supplementary Fig. 15). These findings revealed LeuK2 (6) and LeuK3 (7) as new leucinostatins, as depicted in Fig. 3c. Overall, these results confirm that LcsG, as an NMT, can iteratively catalyze the methylation of NRP terminal amines.
Leucinostatins are well-known antibiotics. We purified these compounds by semipreparative HPLC and successfully generated LeuA0 (8), LeuK0 (4) and LeuK3 (7) in sufficient quantities for the antimicrobial assay. The inhibitory activity of these compounds against the drug-resistant strain C. neoformans H99 and the plant pathogen P. infestans was determined using agar diffusion assays. Notably, previous studies have demonstrated the inhibitory effects of LeuA and LeuB on both C. neoformans and P. infestans. All of these leucinostatins had inhibitory effects on these pathogens (Fig. 3d). Moreover, the anti-C. neoformans MICs value of the two methylated products, LeuA0 (8, 25.8 μg/ml) and LeuK3 (7, 25.8 μg/ml), were four and two times lower than those of their parent products, LeuA (3, 102.4 μg/ml) and LeuK0 (4, 51.2 μg/ml), respectively (Supplementary Fig. 16), which indicated that N-methylation at the terminus of leucinostatins can improve their antimicrobial efficiency.
We then identified the catalytic residues in LcsG. Local multiple sequence alignments revealed that LcsG shares a conserved SAH/SAM-binding motif (D296, D321, D348, and K363) and two possible catalytic residues (D368 and D395) (Supplementary Fig. 17a). Despite many attempts, we were unable to obtain a crystal of the LcsG protein suitable for X-ray crystallographic analysis. As an alternative, we employed an artificial intelligence (AI) method, Uni-Fold, to approximate a model of LcsG. The overall structure of LcsG with color-coded pLDDT scores is presented in Supplementary Fig. 18a, and the six predicted residues are depicted in Supplementary Fig. 18b. Additionally, Supplementary Table 2 provides the pLDDT scores for these six predicted residues. These results indicated that AI predicts the protein structure with high confidence, including the active site residues that are proposed to be relevant for substrate binding and catalysis. Structural alignments between LcsG and its homologs are shown in Supplementary Fig. 17b, with corresponding RMSD values detailed in Supplementary Table 3. The overall structure of LcsG involved a typical Class I methyltransferase fold; the N-terminus appears to be responsible for dimerization and substrate binding and the C-terminus appears to be responsible for SAM-binding (Supplementary Fig. 18b). To determine the structure‒function relationship of LcsG, we conducted a molecular docking analysis using the predicted LcsG structure and SAH. SAH was docked into the LcsG structure model binding pocket, and the hydrogen bond network that mediates SAH binding was present in the final docking position.
As shown in Fig. 4a, potential hydrogen bond interactions between SAH and residues D296, D321, D348, and D363 were detected, which was consistent with the results of multiple sequence alignment (Supplementary Fig. 18c). The accurate stereo-structure structures of nonpeptide leucinostatins are difficult to predict because they are composed of seven nonstandard and unusual α-amino acid residues. Based on the crystal structures of the analogs helioferin A and ZHAWOC6027 (Supplementary Fig. 19), a structural model of LeuA was predicted, and LeuA was docked into the LcsG structure via DiffDock. In the first ranked result, the N atom that becomes methylated occupies the position between D368, D395 and SAH (Supplementary Fig. 20). Subsequent molecular dynamics (MD) simulations were used to further explore the stabilities of our protein‒ligand complex and the importance of catalytic site residues. After 500 ns of equilibration (Supplementary Fig. 21), 3 independent 500 ns MD simulations were performed (Supplementary Table 4 and Supplementary Fig. 22). The results showed that the positively charged N is located between D395 and D368, and the terminal N of LcsG could form a stable complex system with D368 (Fig. 4b). Nevertheless, the results provided insight into the importance of D368. In some snapshots, the ligand formed salt bridges with D368 and D395 (Supplementary Fig. 23).
To verify this result, we mutated D296, D321, D348, K363, D368, and D395 to Ala in LcsG (Supplementary Fig. 24). Biochemical assays of these mutants were then performed using LeuA as substrate. After 1 h of incubation, the conversion of LeuA (3) to LeuA0 (8) decreased for all the mutants (Fig. 4c). These findings are consistent with our earlier in vitro assays and docking and MD simulation results. These findings indicate that the SAM-binding site of LcsG is involved in leucinostatin methylation and provide additional support that LcsG is a SAM-dependent methyltransferase. Regarding the enzymatic mechanism of methyltransferases, numerous studies have proposed a reaction mechanism for OMT, involving a His/Glu dyad and an Asp residue. The Glu residue was placed near the His residue, and the His residue was activated to deprotonate the hydroxyl group in the substrate. The Asp residue was shown to interact with the substrate to improve binding. The deprotonated hydroxyl group functions as a good nucleophile to attack SAM, which is a methyl donor, to form the O-methylation product. In the case of NMT, similar but different mechanisms have been proposed. A QM/MM study on the catalytic mechanism of phenylethanolamine NMT revealed that unlike OMT, a Glu residue was employed to deprotonate the protonated amine in the substrate to form a nucleophile. Then, the methyl group was transferred from the methyl-donor SAM to the deprotonated amine group. Unlike reactions catalyzed by OMT, a His/Glu dyad was not needed for NMT. The lone electron pair of the N atom on the dimethylamine group can undergo a nucleophilic attack. We proposed that the protonated dimethylamine group in leucinostatins might be coordinated and deprotonated by two negatively charged residues (D368 and D395). A nucleophilic attack between dimethylamine and SAM followed, and the methyl group was transferred from SAM to leucinostatins. Compared to wild-type LcsG, the D368A and D395A mutants exhibited markedly obvious decreased methylation (Fig. 4c), indicating that these two residues contribute notably to the substrate binding. For LcsG, the mutation data, docking results and MD simulation results collectively gave a hint that its reaction mechanism is similar to that of phenylethanolamine NMT.