We previously proposed a consensus framework for classification and evaluation of aging biomarkers1. Now, we address biomarker validation as the next step in the clinical translation process. First, we review current efforts to validate predictive biomarkers of aging using population-based cohort studies and discuss challenges encountered during this process. We primarily focus on biomarkers that are blood-based, composite (as opposed to single-molecule markers) and based on ‘omic’ assays. Blood is widely accessible, obtained in a minimally invasive manner, and in constant contact with other tissues, potentially providing information about the biological age of the entire organism (although this is still under active exploration11,12,13). Composite panels of biomarkers are more likely to capture systemic effects of the complex aging process than single biomarkers12,13,14,15,16, and those based on rapidly advancing high-throughput omic technologies and artificial intelligence (AI) are expected to substantially advance the performance and translational value of next-generation biomarkers of aging15. To facilitate and enhance rigor in the validation process17, we provide guidelines for standardization and harmonization of biomarkers across populations with unique characteristics, and we make recommendations on the metrics that should be used to report their predictive performance.
Ideally, a biomarker measure should be robust against random and systematic sources of variability arising from technical and pre-analytical sources or application to different populations. Also, extensive information should be available on covariates to be considered to optimize their performance. We briefly outline some important types of conceptual and technical considerations and terminology important for biomarker validation in Box 1. Overall, a comprehensive process that encompasses multiple types of validation is desirable to establish reliability, accuracy and clinical utility of a biomarker of aging.
To date, predictive validation of aging biomarkers (for their association with age-related outcomes) has mostly relied on data previously collected in observational cohort studies. This process is currently the most active area of research in the aging biomarker validation space as an important prerequisite to further validation and ultimate clinical use. Cohort studies typically collect samples and clinical data on health and functional status at multiple points in time and allow assessment of association and predictivity of biomarkers for multiple health outcomes across different populations as well as the identification of relevant covariates. We focus on cross-population validation (that is, validation in more than one cohort) because it is the most robust approach for validation of blood-based biomarkers of aging in retrospective observational studies. To contextualize recommendations outlined in later sections, we first outline the current state of biomarker validation efforts (including different data sources) and discuss challenges to progression in this field.
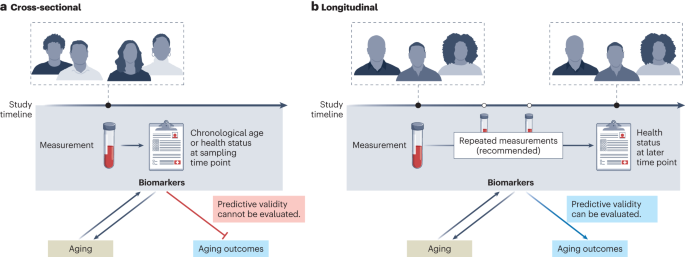
The development of early biomarkers of aging was facilitated by open-access availability of large datasets (such as those stored within the Gene Expression Omnibus), many of which are derived from cross-sectional studies. Cross-sectional studies provide a snapshot in time of variable measurements and corresponding phenotypic data (Fig. 1a). Such studies identified many biomarkers that correlate with chronological age. These include several soluble biomarkers of inflammation (for example, interleukin-6 (IL-6) or C-reactive protein) or hormonal status (such as fasting insulin and dehydroepiandrosterone sulfate). Early ‘first-generation’ epigenetic biomarkers were also used to predict chronological age. However, cross-sectional age associations can be biased by secular trends and selective attrition to study participation, which can preclude assessment of the predictive value of the marker in relation to future age-relevant outcomes. Furthermore, cross-sectional studies do not allow assessment of within-individual changes in response to interventions (sensitivity to change), a key requirement for the use of biomarkers of aging in clinical trials.
In contrast to cross-sectional studies, longitudinal studies collect biological measures (omics or other biomarkers), phenotypes (clinical characteristics) and adverse age-related health outcomes serially over time in the same individuals (Fig. 1b). Most longitudinal studies also include data on genetic variants, and, through Mendelian randomization studies, they may help determine whether specific biomarkers are causally related to health outcomes or rather reflect the activation of mechanisms aimed at counteracting the pathologic processes that lead to those adverse health outcomes (generally defined as ‘resilience’ mechanisms). Most studies collect longitudinal information on participant demographics (for example, age, sex), physiological measurements (for example, body mass index, blood pressure) and routine laboratory results (for example, complete blood count or hemograms or blood biochemistry) and may additionally collect data on mortality and cause of death as well as other aging-associated outcomes including multimorbidity, performance-based measures of physical and cognitive function, and frailty. Measures of disability in activities of daily living and instrumental activities of daily living provide information on a participant’s level of independence but also health deterioration over time.
Analytically, biomarkers are often considered at one point in time and related prospectively to future outcomes, such as disease onset, change in physical and cognitive function over time or mortality. However, a more informative approach is to consider repeated measures obtained from the same participants at regular intervals. This approach allows the study of the relationship between biomarkers and the time trajectories of clinical outcomes, which provide the best approximation of the ‘pace of aging’. Therefore, longitudinal cohort data can uniquely support the development and validation of biomarkers of aging, such as prospective validation against multiple different outcomes and across independent populations. Additional approaches focus on resilience, healthy aging or other aging-related outcomes. Moreover, outcomes related to healthcare resource utilization, such as the rate of hospital admissions and use of emergency rooms, may also be highly relevant. The prioritization of aging-associated outcomes and information on (functional) aging trajectories separate from mortality could make such biomarkers even more appealing for translation to clinical studies.
Many cohort studies establish biobanks that safely store biospecimens that can then be accessed in the future to test new hypotheses or employ newly available technology for analysis. Biobanks are invaluable resources for biomarker research (particularly when it comes to testing and validation), especially if linked clinical and/or omic data and follow-up samples and/or data are available. In addition to the samples collected as part of a standard cohort study with specific research questions, large-scale, general-purpose biobanks also exist and can be useful for biomarker development. For example, the UK Biobank contains in-depth genetic and health information and holds biological samples from half a million UK participants. Multiple studies have already evaluated omic-based predictors of various aging-related outcomes in the UK Biobank. With the decreasing costs of measuring biomarkers, this and other biobanks are currently expanding their range of available omics data. The Finnish FinnGen cohort (n = ~500,000), BioBank Japan (n = ~260,000) and the Mass General Brigham Biobank (n = ~135,000) have also recently generated large multiomics datasets, which are expected to be used to validate multiple biomarkers for aging-related outcomes. Some repositories are taking steps to organize their data in well-documented and accessible databases: for instance, the US National Institute on Aging has launched complementary translational longevity initiatives to generate large-scale, cross-species, multiomics datasets.
Even with existing cohort studies and biobanks, systematic cross-population validation remains relatively limited. Nevertheless, several biomarkers of aging have been tested across multiple cohorts, with the most commonly examined outcome being all-cause mortality. Although there are issues surrounding mortality as an endpoint, it has the advantage of being clearly defined.
A representative list of studies validating blood-based composite biomarkers for prediction of future mortality is shown in Table 1. Many of these studies were conducted by researchers who developed the biomarker and validated that specific biomarker across multiple cohorts or by researchers who used biomarkers developed by others and compared multiple biomarkers within one cohort, with differences between the two approaches illustrated in Fig. 2. In addition, a representative list of biomarkers of aging that have been tested across multiple cohorts is shown in Supplementary Table 1 (cohorts are described in Supplementary Table 2). Our intention here is not to systematically review previous studies or perform a meta-analysis; rather, the studies considered were selected to illustrate the challenges of validating biomarkers of aging in a reliable, comparable and generalizable manner.
In studies validating blood-based biomarkers, most reported hazard ratios (HRs) for prediction of mortality risk are in the moderate range; however, a few studies have reported impressive metrics that render those biomarkers good potential candidates for use in preclinical and clinical studies. For example, Huan et al. and Deelen et al. reported increased mortality risk (HRs of 1.85 and 2.73) for their epigenetic and metabolomic biomarkers, respectively. Nevertheless, these values should be interpreted with caution because they rely on different units of measure; they will need to be substantiated by independent validation in a different cohort, and their performance needs to be compared with other biomarkers using consistent reporting measures. Thus far, relatively few studies have compared individual (composite) biomarkers across multiple cohorts or multiple biomarkers across the same cohort using standardized and equivalent measurement units that make them fully comparable. We argue that studies featuring systematic and comprehensive benchmarking of diverse biomarkers of aging across many large cohorts with extended follow up (>10 years) are needed to substantially advance the field (Fig. 2a).